PREZISTA®/r: Tolerability Proven Over Time
Low Rates of GI ADRs for Once-Daily PREZISTA®/r vs Twice-Daily PREZISTA®/r1
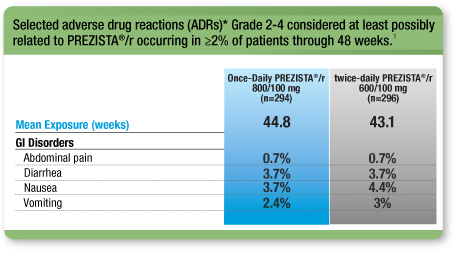
* Excluding laboratory abnormalities.
This is not a complete list of all ADRs reported.
- Low discontinuation rate due to ADRs for Once-Daily PREZISTA®/r vs twice-daily PREZISTA® /r; 3.4% vs 4.7%, respectively1, 2
- No cases of jaundice or scleral icterus were reported in the PREZISTA®/r treatment arm2
Selected Important Safety Information
- Hepatotoxicity: Drug-induced hepatitis has been reported with PREZISTA®/r. During the clinical development program (N=3063), hepatitis has been reported in 0.5% of patients receiving combination therapy with PREZISTA®/r. Patients with preexisting liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities, including severe hepatic adverse reactions
- Severe Skin Reactions: Severe skin reactions (0.4%), accompanied by fever and/or elevations of transaminases in some cases, and Stevens-Johnson Syndrome ( <0.1%) have been reported in patients receiving PREZISTA®/r. During post-marketing experience, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis have been reported in patients receiving PREZISTA®/r. Discontinue PREZISTA®/r immediately if signs or symptoms of severe skin reactions develop (including, but not limited to, severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis, and/or eosinophilia)
Please see additional Important Safety Information.
Please see full description of study design and baseline characteristics.
Selected ADRs (≥Grade 2) from 3 clinical trials1, 2
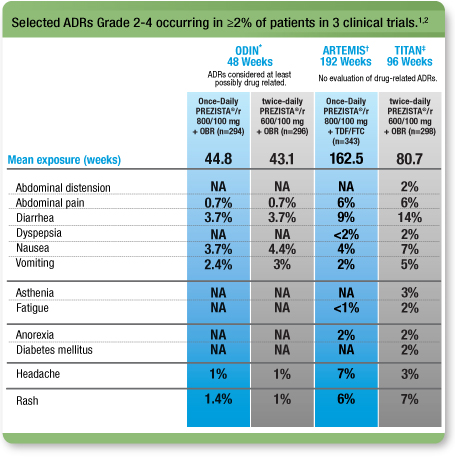
NA=not applicable
This is not a complete list of all ADRs.
* ODIN: A randomized, open-label, Phase 3, 48-week, noninferiority clinical trial comparing Once-Daily PREZISTA®/ritonavir 800/100 mg with twice-daily PREZISTA®/ritonavir 600/100 mg in treatment-experienced adult patients with no DRV RAMs (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V).
† ARTEMIS: A randomized, controlled, open-label, Phase 3, noninferiority clinical trial comparing PREZISTA®/r 800/100 mg once daily (n=343) with Kaletra® 800/200 mg per day (n=346) in treatment-naïve adult patients with HIV-1 RNA ≥5000 copies/mL.
‡ TITAN: A randomized, controlled, open-label, Phase 3 clinical trial comparing PREZISTA®/r 600/100 mg twice daily (n=298) with Kaletra 400/100 mg twice daily (n=297) in less treatment-experienced, lopinavir-naïve adults.
GI=gastrointestinal; ADRs=adverse drug reactions; OBR=optimized background regimen; TDF/FTC=tenofovir disoproxil fumarate/emtricitabine.Kaletra (lopinavir/ritonavir) is a registered trademark of AbbVie Inc.



